Chapter 15, “Environmental Chemistry – II Water,” explores water’s unique properties, its importance in daily life, and the challenges associated with water quality.
From understanding water’s molecular structure to tackling key environmental issues like water pollution and hardness, this chapter is a cornerstone for students aiming to grasp real-world applications of chemistry.
This article will provide additional conceptual questions to solidify your understanding and help in exam preparation.


Topics Covered in These Notes
This section delves into the crucial topics covered in Chapter 15, providing a comprehensive understanding of:
- Water properties and its role as a universal solvent.
- The difference between hard and soft water, and methods of removing hardness.
- Sources and effects of water pollution.
- Key chemical principles like capillary action and hydrogen bonding.
These notes offer detailed answers to all significant questions while addressing common misconceptions.
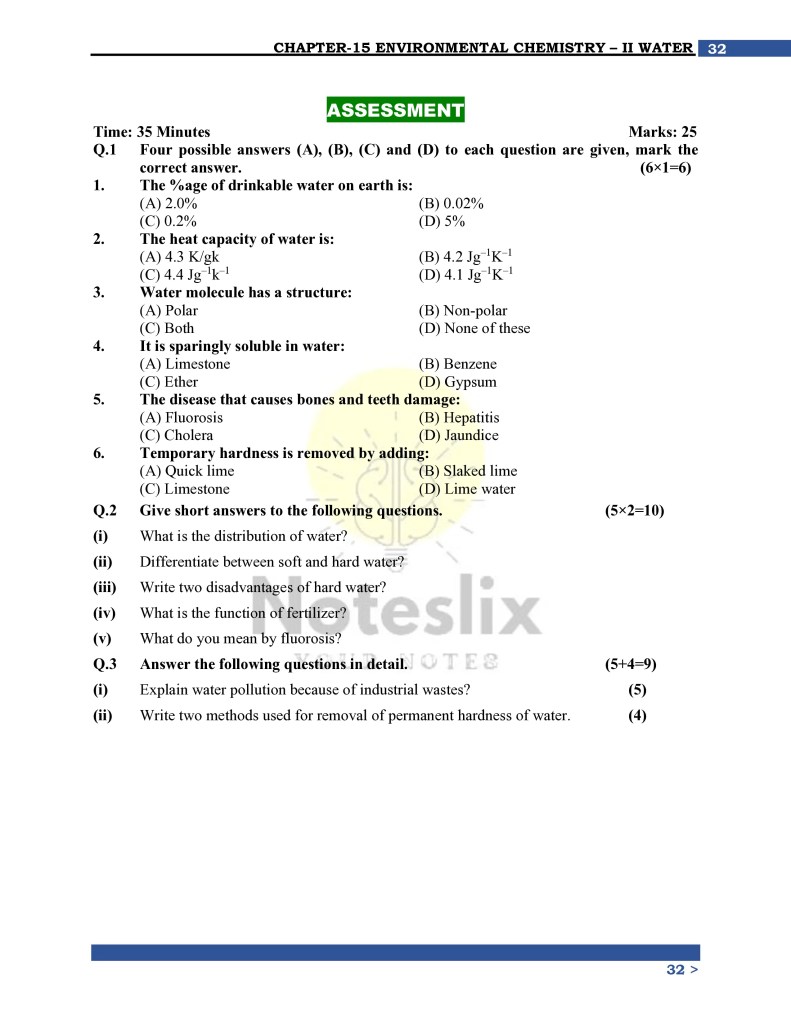
Class 10th Chemistry Chapter 15 Additional Important Conceptual Questions
Below are some critical questions and answers that go beyond standard textbook content, offering deeper insights into the subject:
- What is the heat capacity of water, and why is it significant?
- Answer: Heat capacity refers to the amount of heat required to raise the temperature of 1 gram of water by 1°C. Water’s high heat capacity (4.2 Jg⁻¹K⁻¹) stabilizes Earth’s climate by moderating temperature fluctuations between day and night.
- How does the polarity of water molecules enable it to dissolve substances?
- Answer: Water’s polar nature, with partial positive (H⁺) and negative (O⁻) ends, attracts oppositely charged ions in substances. This interaction overcomes the electrostatic forces in ionic compounds, enabling dissolution.
- What causes the hardness of water, and how is it removed?
- Answer:
- Temporary hardness: Caused by bicarbonates of calcium and magnesium. It is removed by boiling or adding lime water.
- Permanent hardness: Caused by sulfates and chlorides of calcium and magnesium. It is removed using washing soda or sodium zeolite.
- Answer:
- What makes non-polar compounds insoluble in water?
- Answer: Non-polar compounds like benzene and ether lack polar ends, preventing interaction with water’s polar molecules. As a result, they do not dissolve.
These questions not only highlight key concepts but also help students connect theory to practical applications.
Tool for Success in Exams
Understanding the core concepts of Chapter 15 equips students to tackle even the most challenging questions in exams. Additional conceptual questions not only enhance critical thinking but also help identify areas that need improvement.
By practicing these questions, students gain confidence and develop a stronger command of the subject. Comprehensive preparation using these notes ensures better performance in exams.
Colored Notes
To make studying more efficient, the notes for Chapter 15 include color-coded sections. These highlight essential terms, definitions, and examples, making it easier to locate key points at a glance.
Visual aids like diagrams and tables further simplify complex topics, ensuring an engaging and effective learning experience.
Notes Are Free to Use
One of the best aspects of these notes is that they are freely accessible to everyone. Students can use these resources without any financial burden, ensuring equal opportunities for learning. These notes can be printed or saved digitally for easy reference anytime.
Notes Are Mistake-Free
The notes provided for Chapter 15 have been meticulously reviewed to eliminate errors. Each concept is explained with precision, ensuring clarity and accuracy. Mistake-free notes play a crucial role in helping students avoid misconceptions and achieve academic excellence.
Conclusion
Chapter 15, “Environmental Chemistry – II Water,” is fundamental to understanding the chemical and environmental aspects of water. These notes and additional conceptual questions ensure a deeper comprehension of the subject, helping students excel in exams.
From learning about water’s properties to tackling real-world challenges like pollution, this chapter offers valuable insights into both chemistry and environmental science. Make use of these comprehensive and user-friendly notes to strengthen your knowledge and achieve your academic goals.
Other Class 10th Chemistry Important Conceptual Questions
- Class 10th Chemistry Chapter 9 Important Conceptual Questions
- Class 10th Chemistry Chapter 10 Important Conceptual Questions
- Class 10th Chemistry Chapter 11 Important Conceptual Questions
- Class 10th Chemistry Chapter 12 Important Conceptual Questions
- Class 10th Chemistry Chapter 13 Important Conceptual Questions
- Class 10th Chemistry Chapter 14 Important Conceptual Questions
- Class 10th Chemistry Chapter 16 Important Conceptual Questions

