Chemical Equilibrium, the topic of Chapter 9, plays a crucial role in understanding the dynamic balance of chemical reactions. This chapter covers the principles, definitions, and examples of equilibrium systems, emphasizing reversible reactions and dynamic equilibrium.
These solved notes provide an excellent resource for students, addressing exam-focused MCQs, short and long questions, and comprehensive explanations to strengthen conceptual understanding.

























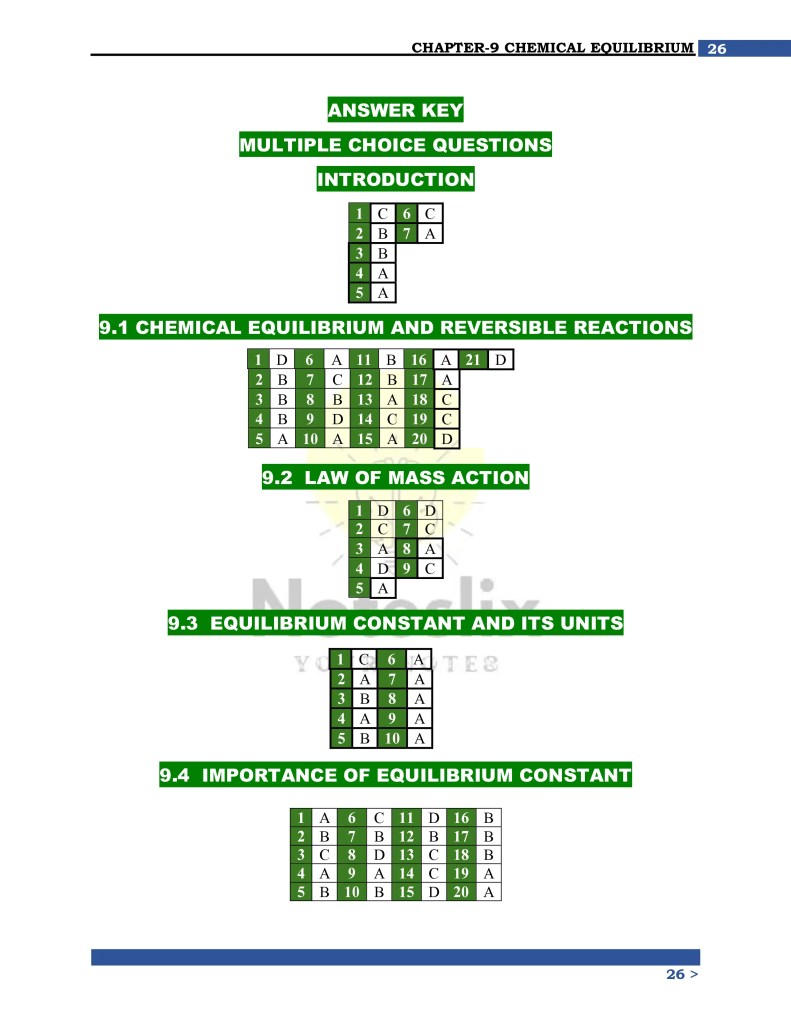
Topics Covered in These Notes
These notes include detailed explanations of the following topics:
- Chemical Reactions and Equilibrium: Definitions and key characteristics of chemical equilibrium.
- Reversible and Irreversible Reactions: Differentiation, examples, and detailed analysis.
- Dynamic Equilibrium: Explanation with graphical representation and real-life examples.
- Law of Mass Action: Its definition, derivation, and applications.
- Equilibrium Constant (Kc): Formula, units, and calculation examples.
- Importance of Kc: Predicting the direction and extent of a reaction.
- Macroscopic Characteristics of Equilibrium: Closed systems, reaction rates, and system properties.
Each topic is supported by solved examples, making the concepts easy to grasp.
Chapter 9 Solved Notes Topic Wise
The notes are meticulously organized into the following categories:
Multiple Choice Questions (MCQs)
These include exam-oriented questions such as:
- Identification of reactants and products in chemical reactions.
- Characteristics of reversible and irreversible reactions.
- Questions related to the Law of Mass Action and dynamic equilibrium.
Short Questions
Important conceptual queries are addressed, such as:
- Differentiation between reversible and irreversible reactions.
- Explanation of static and dynamic equilibrium with examples.
- Significance of equilibrium constant in predicting reaction direction.
Long Questions
These cover detailed explanations with examples:
- The derivation of equilibrium constants for general reactions.
- Characteristics of dynamic equilibrium with graphical representations.
- Comprehensive discussion on the Law of Mass Action.
d. Numerical Problems
Examples include calculating equilibrium constants for reactions, and providing step-by-step solutions for clarity.
Tool for Success in Exams
These solved notes are specifically designed to help students:
- Understand Core Concepts: Detailed explanations ensure conceptual clarity.
- Enhance Problem-Solving Skills: Numerical examples provide practice with step-by-step solutions.
- Ace Exams: The inclusion of MCQs, short, and long questions offers complete exam preparation.
Colored Notes
The notes are designed with colored highlights to:
- Emphasize important points and key formulas.
- Differentiate between topics for better organization.
- Make the material visually engaging and easy to revise.
6. Notes Are Free to Use
These notes are available free of cost, ensuring accessibility for all students aiming to excel in their Chemistry exams. Students can utilize them to improve their understanding without worrying about expenses.
Notes Are Mistake-Free
Accuracy is vital when preparing for exams, and these notes are crafted with the utmost precision. Every answer, definition, and numerical solution has been cross-verified to ensure correctness. This ensures students can rely on these notes without any hesitation while preparing for their exams.
Conclusion
Class 10th Chemistry Chapter 9 solved notes are a comprehensive resource for students. Covering MCQs, short and long questions, and numerical problems, these notes provide an in-depth understanding of Chemical Equilibrium.
With clear explanations, graphical representations, and step-by-step solutions, these notes are a must-have for mastering the chapter and excelling in exams. Their free and mistake-free nature makes them even more accessible and reliable for students aiming for academic success.
Other Class 10th Chemistry Solved Notes Topic Wise
- Class 10th Chemistry Chapter 10 Solved Notes Topic Wise
- Class 10th Chemistry Chapter 11 Solved Notes Topic Wise
- Class 10th Chemistry Chapter 12 Solved Notes Topic Wise
- Class 10th Chemistry Chapter 13 Solved Notes Topic Wise
- Class 10th Chemistry Chapter 14 Solved Notes Topic Wise
- Class 10th Chemistry Chapter 15 Solved Notes Topic Wise
- Class 10th Chemistry Chapter 16 Solved Notes Topic Wise

